


Since its birth in 2020, the IHMD Forum has flourished in an environment where opportunities and challenges coexist. After four years of unremitting efforts, the scale of the forum has increased by over 200%, the number of sponsors has increased by 8 times, the overall scale of participation has increased by 7.5 times, and the retention rate of old customers has exceeded 70%. It has become an influential forum for China to intervene in the field of high-end medical devices.
grown in size
grown in sponsors
grown in scale
retention regular customers

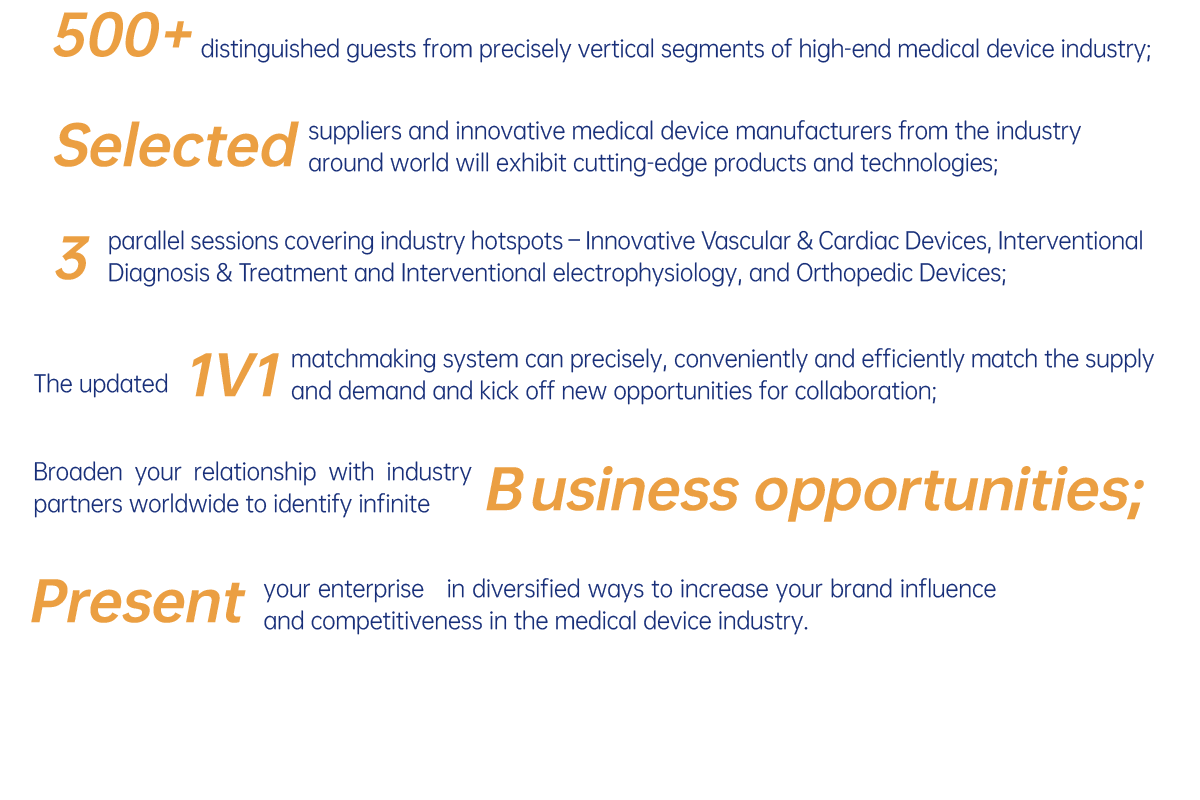
Themed "Achieve Win-win through Innovation-driven Collaborations" the 6th International High-end Medical Device Forum (IHMD 2025) will take place in Beijing from March 27th to 28th. Since its inception, IHMD has witnessed the flourish and profound transformation of the medical device industry. Today, new technologies and ideas continue to spring up in the medical device sector, bringing unprecedented opportunities and challenges to the industry. At the same time, driven by the evolving needs from domestic and international markets, enterprises are leaning into reordering their strategies to adapt to the new market landscape.
IHMD 2025 features three major forums: Innovative Vascular & Cardiac Devices, Interventional Diagnosis & Treatment and Interventional Electrophysiology, and Orthopedic Devices. These forums will delve into the status quo, trends, opportunities, and challenges of the industry, providing comprehensive insights from multiple dimensions including technological innovations, clinical applications, market demands, and industry policies, to facilitate exchange of ideas, cooperation, and common development.
Attendees will discuss how to expand the future landscape of the medical device industry in the new era driven by innovation. By sharing experiences, exchanging ideas, and collaborating on innovation, we aim to facilitate the higher-level development of the industry and make greater contributions to the cause of human health.
IHMD 2025 looks forward to advancing with you!

Cardiovascular and cerebrovascular diseases, electrophysiology, endoscopy, imaging, nerve intervention, surgical robot, medical beauty, orthopedic implant, drug-eluting balloon, cardiovascular intervention, biological valve, surgical robot, hospital/scientific research institution, government/school.


Medical materials, process services (surface treatment and sterilization), processing and manufacturing equipment, equipment accessories and consumables, pipe fittings and extrusion processing, electronic components, components and accessories, plastic film forming services and equipment, packaging, printing, labeling, contract manufacturing services, testing, measuring, inspection and calibration equipment and supplies, medical manufacturing automation, consulting services, electronic components, wire harnesses, accessories and finishing, and others.



Plenary Session: "Achieve Win-win through Innovation-driven Collaborations"
Innovative Vascular & Cardiac Devices Forum
Interventional Diagnosis & Treatment and Interventional Electrophysiology Forum
Innovative Vascular & Cardiac Devices Forum
Orthopedic Devices Forum
Innovative Vascular & Cardiac Devices Forum
Orthopedic Devices Forum

























